CROSS-REACTIVITY IN THE IMMUNE SYSTEM: SIN OR VIRTUE?
By: Adhella Menur
How the adaptive immune response memorized the pathogen attack
The human immune system resembles a top-tier orchestra, capable of playing anything from the simple “Twinkle-Twinkle Little Star” to the challenging Beethoven’s “Symphony No. 9, also known as The Choral Symphony.” Each instrument plays its role harmoniously to deliver an optimal performance. Even a similar symphony played by this orchestra deserves a standing ovation.
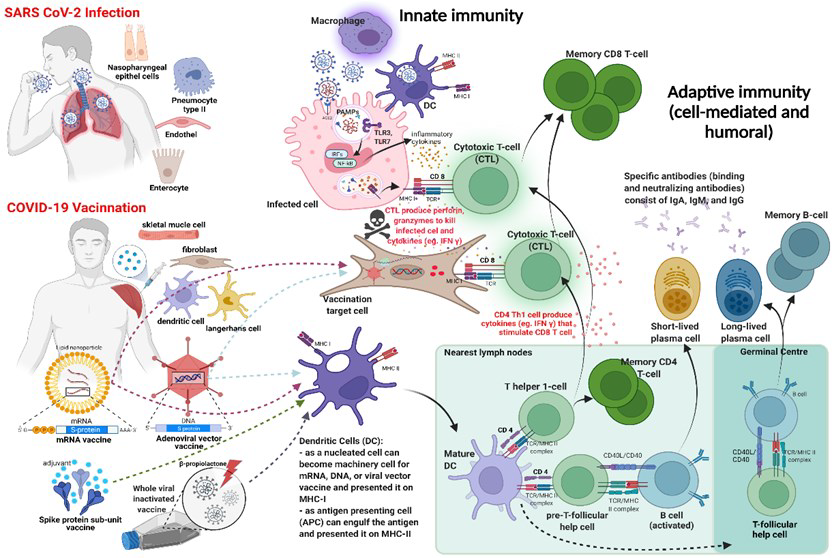
In the human immune system, every immune cell plays a significant role in collectively defending against various pathogens. This complex mechanism can be simplified into having two “lines of defense”: innate immunity and adaptive immunity. Innate immunity serves as the first line of defense against pathogens, consisting of four types of defensive barriers: anatomical (skin and mucous membranes), physiological (temperature, low pH, and chemical mediators), endocytic and phagocytic, and inflammatory responses. It provides immediate, non-specific defense responses, involving cells of both hematopoietic and nonhematopoietic origins, relying on pattern recognition receptors (PRRs) to detect pathogen-associated molecular patterns (PAMPs). Hemato-poietic cells involved in innate immune responses include phagocytes (macrophages and neutrophils), eosinophils, basophils, dendritic cells, mast cells, natural killer (NK) cells, and NK T-cells. Upon encountering pathogens, innate immune cells rapidly migrate to infection sites and promote inflammation by producing cytokines and chemokines for clearance. Phagocytes engulf pathogens and activate the adaptive immune response by mobilizing and activating antigen-presenting cells (APCs).
Subsequently, the innate immune system aids the action of the adaptive immune system, mainly when innate immunity is ineffective in eliminating pathogens. The adaptive immune system is based on the clonal selection of lymphocytes with anti-gen receptors: effector T-cells (cytotoxic T-cells and helper T-cells) which are the cornerstone of cell-mediated immunity and B-cells, which involve in humoral-mediated immunity via differentiation into plasma cells to produce antibodies. T and B cells are activated when they recognize small components of antigens, called epitopes, presented by APCs. Then, they develop a special feature of adaptive immunity, immunologic memory, to record and store their experiences with various pathogens. After the activated T-cells undergo expansion and contraction and initiate the memory pool, 90-95% undergo apoptosis, and the remaining 5-10% differentiate into memory T-cells. Then, memory T-cells form central memory T-cells (Tcm), effector memory T-cells (Tem), tis-sue-resident memory T-cells (Trm), regulatory T-cells (mTreg), and stem memory T-cells (Tscm). They circulate in the blood and persist in secondary lymphoid organs, like the spleen and lymph nodes. Memory B-cells can be formed in two T-cell-dependent mechanisms: they differentiate into short-lived plasma cells, and in the second, they are formed and differentiated in dependent or independent germinal centers (GCs) of peripheral lymphoid organs. They reside mainly in secondary lymphoid organs and undergo affinity maturation, where their antibodies acquire increased specificity and affinity for the particular pathogen. This enables the immune system to mount efficient and swift responses when re-encounters the same or similar pathogens. Vaccination relies on the principle that introducing known antigens pathogens triggers a primary immune response. While the vaccine may not experience this response as an illness, it still confers immune memory.

Cross-reactivity in the immune system: The Sin
In our daily lives, we encounter various pathogens and survive thanks to the remarkable features of the adaptive immune system, which recognizes and memorizes diverse antigens, thus generating specific immunities. However, the picture becomes incomplete when we consider cross-reactivity in the immune system. Cross-reactivity is expected in both T and B-cells due to the presence of many overlapping epitopes on antigen surfaces and the limited diversity of unique human B-cell receptor (BCR) and T-cell receptor (TCR) clonotypes needed to maintain immunity against various pathogens. Cross-reactivity occurs when two distinct epitopes share structural similarities and are recognized by immune memory cells. This phenomenon has significant consequences for both hosts and pathogens in terms of host health, antigenic variation, and epidemiological dynamics. It can provide cross-protection preventing immune escape and aiding vaccine strategies. However, it can also have detrimental effects by inducing or amplifying disease pathogenesis and immunopathology. The question remains: Is cross-reactivity a strategy to combat pathogens across antigenic space, or is it an unintended consequence of the immune system’s work in the vast ocean of antigenic variation—sin or virtue?
When discussing cross-reactivity as a sin, we encounter the term “original antigenic sin” (OAS). This term was proposed by Thomas Francis Jr. in 1960, referring to the original sin committed by Adam and Eve in the Bible. The original sin is a Christian view that arose from Adam and Eve’s transgression in Eden, the sin of disobedience in eating the forbidden fruit from the tree of the knowledge of good and evil. The sin is then imprinted and is passed on to all future generations. In Francis Jr.’s observation, he noticed that influenza antibody titers, as determined in the haemagglutination inhibition (HI) assay, were highest against those influenza strains to which specific age cohorts had first been exposed. For example, people infected with H1N1 influenza viruses during childhood (and thus imprinted with that set of epitopes) were protected later in life against infection with a related virus such as H5N1 but not infections with more distantly related H3N2. The basis for the original antigenic sin thus is imprint-ing: the phenomenon where the first exposure to an antigen shapes the immune response to subsequent exposures to related antigens that have a mixture of shared previously encountered and new epitopes.
The mechanism of OAS occurs when the body is re-exposed to a slightly evolved or different pathogen during a subsequent exposure but has similar epitopes from the first exposure. Still, the immune memory cells process it based on their memory storage with a focus on the imprinted antigen. As a result of the cross-reactivity, the immune system is thus able to respond to the intrusion more robustly and quickly. However, the problem arises when the new antigen is sufficiently different from the imprinted antigen, and the response to the new antigen is not quite precise, leading to a less effective response and possibly failure to clear the pathogen. In such a scenario, not only can the memory response be ineffective, but it can also hinder naïve activated immune cells from differentiating and neutralizing in responding to new epitopes.

The detrimental impact of cross-reactivity in the immune system is when the response toward the original or primary antigen by immune memory cells dominates and disrupts the production of high-avidity de novo immune cell responses. Cross-reactive T-cells may bind with lower affinity to epitopes from the subsequent pathogen compared with the first. Low-affinity epitope binding by cross-reactive CD8+ T cells may lead to immunopathology and reduce viral clearance. Low-avidity cross-reactive antibodies or low levels of high-avidity cross-reactive antibodies that cannot neutralize a subsequent pathogen may even aid the pathogen entry into cells through Fc-receptor-mediated endocytosis, known as antibody-dependent enhancement (ADE).
The OAS resulting in ADE phenomenon is well recognized in the secondary dengue virus (DENV) infection with different serotypes. Antigen-specific antibodies provide long-lasting protection against re-infection with the same DENV serotype but are not protecting enough against other serotypes. In that case, previous DENV serotype antibodies occupy most of the immune response due to immunological memory, thus preventing the development of a new response to different serotypes. Unable to neutralize the virus, the cross-reactive antibodies facilitate viral uptake to cells and enhance viral replication associated with severe DENV infection. Due to its high similarity with DENV, it is also probable that in previously DENV-infected individuals, Zika virus (ZIKV) infection will trigger the production of non-neutralizing antibodies or ineffective T-cell responses. OAS also had been the cause of a significant setback for the first Respiratory Syncytial Virus (RSV) vaccine development in 1960. When the vaccine was administered to RSV-naive newborns who later became naturally infected with RSV, a high proportion had severe respiratory disease, with fatal outcomes in some cases. Another example of OAS is that pre-existing immune responses to other microorganisms might have skewed anti-Plasmodium-specific T-cell repertoire to a non-specific and non-protective response against malaria.

Cross-reactivity in the immune system: The Virtue
In reality, cross-reactivity in the immune system has two sides: it can be detrimental when the response to a non-protective epitope dominates, as explained earlier, or it can be desirable when the response to shared epitopes results in successful neutralization. Under certain circumstances, cross-reactivity in the immune system can simultaneously protect the host against a diverse range of pathogens. Beneficial cross-reactive T-cells can be generated through the priming of T-cells by high-homology epitopes. These T-cells can then cross-react with high avidity during a subsequent infection with a related or similar pathogen, potentially aborting the infection or expediting pathogen clearance by forming a ‘secondary-like’ memory immune response with an increased magnitude of B-cell and T-cell responses. Beneficial cross-reactive memory B-cells and antibodies can be generated against epitopes with high similarity between a primary and a new subsequent infection. Cross-reactive B cells specific to highly conserved epitopes may produce an expedited and highly functional memory-like response to heterologous infection, including the production of cross-neutralizing antibodies.
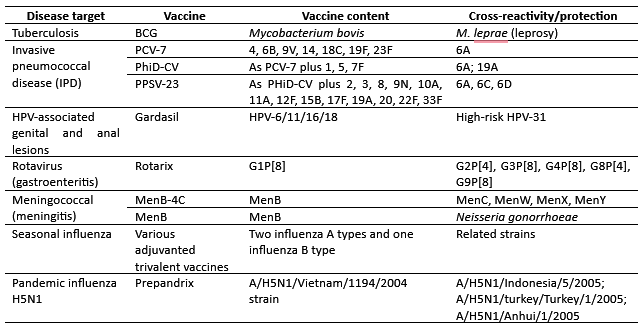
The most famous example of the virtue of cross-reactivity is the discovery by Edward Jenner in 1796 that inoculation with cowpox protected individuals from the related but deadly smallpox virus — leading to the eventual eradication of smallpox and the foundation of the field of vaccinology. Another example includes cross-reactive antibodies from a person infected with Plasmodium vivax, which can inhibit the growth of Plasmodium falciparum in vitro. Moreover, cross-reactive antibodies benefit people living in endemic areas of multi-strain malaria transmission. Additionally, the Jap-anese encephalitis (JE) vaccination has been shown to induce cross-reactive antibodies to West Nile virus in humans. Vaccinologists aim to achieve the virtue of cross-reactivity in the form of ‘‘cross-protection”, which implies clinically significant protection against infection or disease due to an immune response elicited against a related pathogen.
There is an intriguing yet debatable hypothesis regarding the role of cross-reactivity in SARS-CoV-2 infection and COVID-19 vaccines in relation to other pathogens. Since the beginning of the COVID-19 pandemic, pre-existing serum antibodies and B and T-cell responses capable of recognizing SARS-CoV-2 have been detected in naive, unvaccinated individuals. These responses may be shaped by prior infections with SARS-CoV-1, seasonal human coronaviruses (HCoVs), paramyxoviruses, and even certain endemic pathogens such as DENV and Plasmodium spp. There is a complex and dynamic interplay of cross-reactivity of those pathogens with SARS-CoV-2 infection and COVID-19 vaccines due to sequence homology, especially in the Spike region, which may have positive clinical and epidemiological impacts.
After three years of the COVID-19 pandemic with substantial circulating virus variants and rapid deployment of multiple vaccines, the worrying negative OAS effect, fortunately, has not been clearly observed. In the “Spotlight” section of Trends in Immunology Journal, Pillai S intrigued the readers with the title “SARS-CoV-2 vaccination washes away original antigenic sin”. He discussed Röltgen et al. findings about Spike vaccination generating greater antibody breadth than natural SARS-CoV-2 infection. Vaccination results in GCs B-cell responses and generates immunological breadth with antibodies that bind viral variants. In contrast, COVID-19 from SARS-CoV-2 infection disrupts GCs and sustains immune imprinting, resulting in limited immunological breadth. Interestingly, current evidence suggests that cross-reactive immunities form part of the immune response to SARS-CoV-2, alongside the de novo response. Data from B-cell fate-mapping experiments showed that secondary GCs are composed of over >90% of naïve B-cells; hence, there would be no competition for antigen between the higher affinity BCR of memory B-lymphocytes with the germline BCR on naïve B-lymphocytes. Kaku et al. assessed the Spike B-cell response in ancestral mRNA-vaccinated donors with Omicron breakthrough infection. Even though during the acute phase, antibodies had a bias towards recognition and neutralization of the ancestral SARS-CoV-2 strain (the OAS effect), the Omicron breakthrough infection then led to a shift in B-cell immunodominance in targeting the novel RBD. Therefore, although measurable immune responses may be negatively impacted by immune imprinting, this does not translate into diminished protection against infection.
Closing Thoughts
In the end, there is no definitive answer regarding whether cross-reactivity in the immune system is a sin or a virtue. Cross-reactivity may represent humans’ adaptation to an unpredictable world of antigenic exposures. It is influenced by factors such as antigenic structures, kinetics (the speed and magnitude of memory versus naive B and T-cell responses), affinity and functionality of the immune response, and the immunological breadth of the response at the time of exposure. If boosting imprinted memory against original antigens does not disrupt the immune response to novel epitopes, it is not a sin but a virtue.
Since 2012, a more neutral term that does not carry a negative biblical connotation has been introduced to describe cross-reactivity or boosted memory responses toward pathogens encountered earlier in life as “antigenic seniority.” This term encompasses both the positive aspects (e.g., broad protection, back-boost) and the negative contributions of past exposures to the immune response toward new exposures (e.g., imprinting, antigenic interference).
Cross-reactivity may hold the key to developing vaccines against highly antigenically and genetically diverse pathogens and preparing for the next uninvited pandemic. Quantifying antigenic distances between pathogens of interest to predict the impact of cross-reactivity could provide insights into potential vaccine strategies. Continued and meticulous research in immunology remains an absolute necessity.
Further reading
- Aguilar-Bretones, M., et al, 2023. Impact of anti-genic evolution and original antigenic sin on SARS-CoV-2 immunity. The Journal of Clinical Investigation, 133(1).
- Brown, E.L. and Essigmann, H.T., 2021. Original antigenic sin: the downside of immunological memory and implications for COVID-19. MSphere, 6(2).
- Duan, L.J., et al, 2022. SARS-CoV-2 vaccine-induced antibody and T cell response in SARS-CoV-1 survivors. Cell Reports, 40(9).
- Fairlie‐Clarke, K.J., et al, 2009. Why do adaptive immune responses cross‐react?. Evolutionary Applications, 2(1).
- Kaku C.I., et al, 2022. Recall of pre-existing cross-reactive B cell memory following Omicron BA.1 breakthrough infection. Sci Immunol.
- King, S.M., et al, 2023. First impressions matter: immune imprinting and antibody cross-reactivity in influenza and SARS-CoV-2. Pathogens, 12(2).
- Kundu, R., et al, 2022. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nature Communications, 13(1).
- Marshall, J.S., et al, 2018. An introduction to immunology and immunopathology. Allergy, Asth-ma & Clinical Immunology, 14(2).
- Pillai, S., 2022. SARS-CoV-2 vaccination washes away original antigenic sin. Trends in Immunology.
- Reincke, S.M., et al, 2022. Antigenic imprinting in SARS‐CoV‐2. Clinical and translational medicine, 12(7).
- Rijkers, G.T. and van Overveld, F.J., 2021. The “original antigenic sin” and its relevance for SARS-CoV-2 (COVID-19) vaccination. Clinical Immunology Communications, 1.
- Rijkers G, et al, 2023. Christ Bearing the Cross: the original antigenic sin of the immune system and its potential role in emerging diseases. Qeios, CC-BY 4.0.
- Vatti, A., et al, 2017. Original antigenic sin: a comprehensive review. Journal of autoimmunity, 83.
- Vojtek, I., et al, 2019. Would immunization be the same without cross-reactivity?. Vaccine, 37(4).
