By: Eka Windari R., Nur Latifah Hanum, Restu Amalia Mukti
Following the Site Closeout Visit conducted on February 19, 2025, the InVITE study activities involving the study teams and subjects at each study site have been officially completed. Currently, the remaining activity is the preparation of the Final Report, which will include comprehensive information covering the entire activities of the InVITE study—from the beginning to its completion.
The Final Report will contain details on the Background, Study Design, Study Workflow, Ethics Approval History, Monitoring Activities, Study Specimen Accountability, Subject Status, Study Results with corresponding discussions (including infor-mation on any publications in international jour-nals based on the study results), Subject Safety Reports, a List of Protocol Deviations, and the Conclusion. The Final Report’s completion target is by the end of April 2025. It will be submitted in May 2025 as a supporting document for the study closure notification to the hospital directors at each study site and the RSUD Kabupaten Tangerang Ethics Committee.
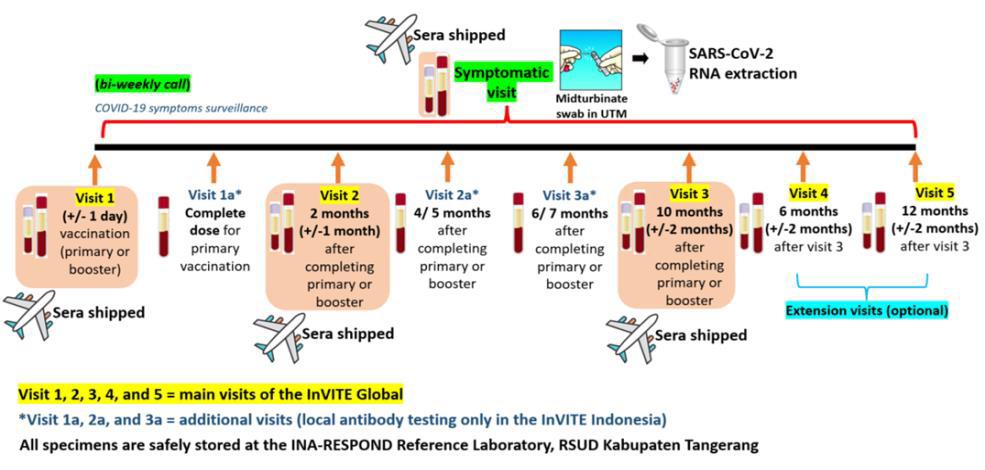 In addition, following the successful shipment of participants’ sera from Visits 1–3 and Symptomatic Visits, the planned shipment of the remaining In-VITE specimens is still in progress. This shipment includes SARS-CoV-2 RNA extracted from mid-turbinate swab specimens collected in Universal Transfer Media (UTM) from Symptomatic Visits and serum specimens from extension Visits 4 and 5. The Material Transfer Agreement (MTA) for this shipment is handled separately from the previous shipment, as Visits 4–5 and some Symptomatic Visits were still ongoing at the time of the initial MTA process. Additionally, the shipment plan was revised due to regulations concerning potential poliovirus in biological specimens—from transporting mid-turbinate swabs in UTM to shipping extracted RNA instead. A new MTA Committee and updated regulations have since been established, including a streamlined review process through the official MTA submission website. However, as the website is currently under maintenance, the required MTA documents—including the draft agreement and appendices—were submitted via email on March 20, 2025. The submission is currently under review by the MTA Committee, and the study team is maintaining active communication with the MTA Secretariat. The team hopes to receive approval soon, as the laboratory testing at the Central Laboratory must be completed by the end of 2025, requiring the shipment to proceed as scheduled in June 2025.
In addition, following the successful shipment of participants’ sera from Visits 1–3 and Symptomatic Visits, the planned shipment of the remaining In-VITE specimens is still in progress. This shipment includes SARS-CoV-2 RNA extracted from mid-turbinate swab specimens collected in Universal Transfer Media (UTM) from Symptomatic Visits and serum specimens from extension Visits 4 and 5. The Material Transfer Agreement (MTA) for this shipment is handled separately from the previous shipment, as Visits 4–5 and some Symptomatic Visits were still ongoing at the time of the initial MTA process. Additionally, the shipment plan was revised due to regulations concerning potential poliovirus in biological specimens—from transporting mid-turbinate swabs in UTM to shipping extracted RNA instead. A new MTA Committee and updated regulations have since been established, including a streamlined review process through the official MTA submission website. However, as the website is currently under maintenance, the required MTA documents—including the draft agreement and appendices—were submitted via email on March 20, 2025. The submission is currently under review by the MTA Committee, and the study team is maintaining active communication with the MTA Secretariat. The team hopes to receive approval soon, as the laboratory testing at the Central Laboratory must be completed by the end of 2025, requiring the shipment to proceed as scheduled in June 2025.

