SINGLE-DOSE HPV VACCINATION: A POTENTIAL SOLUTION TO BOOST GLOBAL CERVICAL CANCER ELIMINATION EFFORTS
By: Restu Amalia Mukti, Adhella Menur

Cervical cancer has a significant impact on women and families in Indonesia, with over 103 million women aged 15 and older at risk. It is the second most common cancer among women after breast cancer, with approximately 36,964 new cases diagnosed annually. Alarmingly, around 70% of cases are detected at advanced stages, when treatment is less effective, contributing to a high mortality rate of approximately 21,000 deaths in 2020. These deaths are largely preventable, as strong evidence shows that cervical cancer is one of the most well-understood cancers in terms of etiology and pathogenesis and is treatable when detected early and managed effectively. The primary cause of cervical precancer and cancer is persistent infection with high-risk (oncogenic) human papillomavirus (HPV) types. There are 12 high-risk HPV types: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. Two of these, HPV 16 and 18, are responsible for approximately 70% of all cervical cancer cases. Years of data have shown that the HPV vaccine is both safe and effective in preventing HPV infections and reducing the incidence of cervical cancer.
In 2020, the World Health Organization (WHO) launched a global initiative to eliminate cervical cancer, aiming to reduce its incidence to 4 per 100,000 women. To achieve this, WHO set the ’90-70-90′ targets: vaccinating at least 90% of girls with the HPV vaccine by age 15, screening at least 70% of eligible women using effective and accessible methods and ensuring at least 90% of women diagnosed with cervical disease receive timely and appropriate treatment. Aligned with this initiative, Indonesia aims to achieve 90% HPV vaccination coverage among 15-year-old girls by 2027, with boys included between 2028 and 2030. The registered HPV vaccines in Indonesia include Cervarix® (bivalent), Gardasil® (quadrivalent), NusaGard® (quadrivalent), and Gardasil®9 (nonavalent). Moreover, 75% of women aged 30 to 69 are targeted for HPV DNA testing, and 90% of those diagnosed with pre-cancerous lesions or invasive cancer should receive treatment by 2030. This strategy is projected to save an estimated 1.2 million lives from cervical cancer by 2070.
As HPV vaccination plays a crucial role in eliminating cervical cancer, we will discuss the single-dose regimen as a potential solution to overcome vaccination challenges.
How the single-dose regimen became a solution to vaccine challenges
In 2006, WHO recommended a three-dose HPV vaccination regimen for adolescent girls (ages 10–19). This was revised in 2014 to a two-dose schedule for girls aged 9–14. Despite these updates, HPV vaccine uptake remains suboptimal, particularly in Low- and Middle-Income Countries (LMICs), where most cervical cancer cases and deaths occur. In 2022, the global completion rate for girls aged 9–14 was just 17%. Challenges include high program costs, supply constraints, inadequate infrastructure, and logistical difficulties in administering multiple doses. Vaccine hesitancy and poor adherence to multi-dose schedules further hinder implementation, with long intervals between doses increasing the risk of losing track of recipients.
Although HPV vaccines contain “virus-like particles” (VLPs) that mimic the outer shell of HPV virions, they are classified as subunit vaccines because they consist of a single purified protein—the L1 major capsid protein—and are entirely non-infectious. Typically, protein subunit vaccines require multiple doses to compensate for weaker initial immune responses and limited antibody production. Additional doses help reinforce and prolong immunity by repeatedly stimulating the immune system, ensuring long-term protection. Given this, how did the idea of a single-dose HPV vaccine emerge?
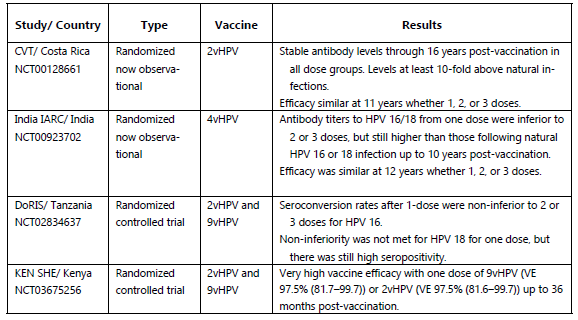
The Costa Rica HPV Vaccine Trial (CVT), a phase 3 randomized clinical trial, first suggested that a single dose might provide protection against HPV16/18 infections. Although the trial aimed to administer three doses to all participants, 20% of women did not complete the full regimen, primarily due to pregnancy or colposcopy referral. The study demonstrated that a single dose of the HPV vaccine provided comparable efficacy (82.1%) against HPV 16/18 prevalent infection to three doses (80.2%) for at least 11 years post-vaccination, with higher effectiveness observed in individuals vaccinated at a younger age. While a single-dose HPV vaccine shows slightly lower effectiveness in preventing HPV16, HPV18, and hrHPV infections compared to multi-dose regimens, its impact on reducing pre-cancer incidence appears to be comparable.
A similar conclusion emerged from the IARC India trial, which began in 2009 to compare the efficacy of two versus three doses of the quadrivalent HPV vaccine in preventing persistent infection and cervical neoplasia. Originally designed as a randomized trial with 20,000 participants, the study was suspended in 2010 due to external factors, leading to an observational cohort with varying vaccine doses, including a significant number who received only a single dose. The trial demonstrated that vaccine efficacy remained consistent across one, two, or three doses over 12 years. Although antibody titers were lower in single-dose recipients than those who received multiple doses, they remained stable for up to 10 years post-vaccination and exceeded levels observed after natural infection.
The biological theories behind the effectiveness of single-dose HPV vaccines stem from their VLP structure, mimicking the key structural features of the actual virus, which strongly stimulates immune cells to produce durable, long-lasting antibodies. Although fewer neutralizing antibodies are generated after just one dose, protection remains effective due to additional immune mechanisms—such as non-neutralizing antibodies that facilitate viral clearance by triggering processes like antibody-dependent phagocytosis, activation of innate immune cells, and complement pathways. These combined immune responses effectively clear HPV even at lower antibody levels. Additionally, HPV’s requirement for epithelial disruption to establish infection gives vaccine-induced antibodies ample time to neutralize the virus.
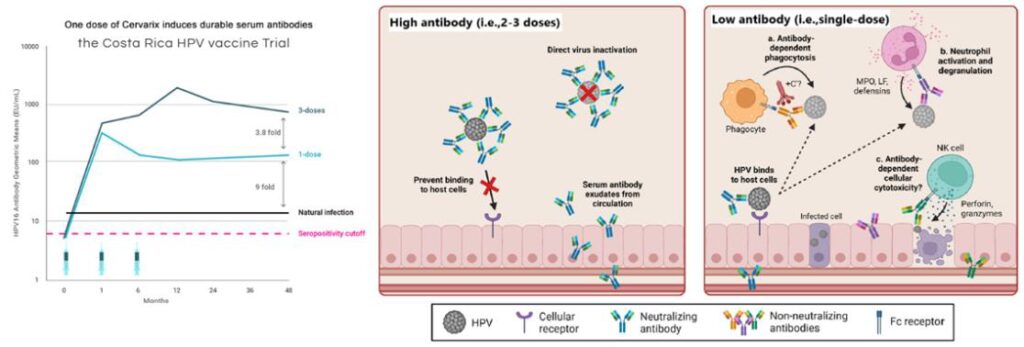
In 2022, the WHO endorsed a single-dose HPV vaccine schedule as an alternative off-label option for girls and boys aged 9–20. This single-dose regimen is expected to be cost-effective, particularly in LMICs, by simplifying logistics, reducing costs, and facilitating the expansion of HPV vaccination programs. It may also encourage countries without national HPV programs to adopt this vaccination. Additionally, a single-dose strategy enables broader coverage by expanding vaccination efforts beyond a single-age group to multiple cohorts.

Single-dose HPV vaccine regimen in Indonesia?
Globally, 141 countries have integrated the HPV vaccine into routine national immunization programs, with 59—including South Africa, Canada, the United Kingdom, and Australia—adopting a single-dose policy. This aligns with WHO recommendations, highlighting that a single dose provides comparable protection to the traditional two-dose regimen.
While Indonesia has not yet transitioned to a sin-gle-dose HPV vaccination schedule, the country remains committed to WHO’s cervical cancer elimination targets. The WHO and the United Nations Population Fund (UNFPA) commend Indonesia’s efforts and stand ready to support adopting a streamlined single-dose approach.
Hopefully, Indonesia will adopt a single-dose HPV vaccine schedule, reinforcing its commitment to women’s health and accelerating progress toward the global goal of eliminating cervical cancer as a public health priority.
Future directions
The long-term protection provided by a single-dose HPV vaccine requires ongoing monitoring. Current research suggests at least 12 years of immunity post-vaccination, but further studies are needed to confirm lifelong efficacy.
A key study investigating this is the ESCUDDO trial (NCT03180034), which compares the effectiveness of a single dose of the bivalent or 9-valent HPV vaccine to the two-dose regimen, with the final analysis expected in late 2025. While preliminary findings are encouraging, robust evidence is still needed. Longer study periods and additional research are necessary to draw definitive conclusions on the long-term effectiveness of this vaccination strategy.
Despite its advantages, a single-dose schedule also raises concerns, particularly in countries with a high HIV burden. Special attention must be given to adolescent girls who may contract HIV after receiving the HPV vaccine, as their immune response and long-term protection may differ. Currently, the WHO recommends that immunocom-promised individuals, including those with HIV, receive a two or three-dose HPV vaccination regimen. Addressing these considerations through further research will be crucial in determining the viability of a single-dose vaccination approach for all populations.
To eliminate the burden of cervical cancer, HPV vaccination must be complemented by accessible cervical cancer screening and timely treatment, along with broader investments in women’s health. That said, ensuring HPV vaccination for every girl represents a significant step forward. Beyond lowering cancer risk, this effort empowers women to lead healthier lives, pursue careers, support their families, contribute to communities, and strengthen their nations—yielding immeasurable societal benefits. Save a Girl, Empower a Nation!
References
- Fokom-Defo V, et al. TitleUnknown. 2024. doi: 10.1016/S2214-109X(24)00009-3
- Gates Foundation. The Most Awesome Explanation About Single-Dose HPV Vaccine [Internet]. [n.d.]. Available from: https://www.gatesfoundation.org/ideas/articles/hpv-single-dose-vaccine-explained.
- HPVWorld. Single-Dose Efficacy Using the Bivalent HPV Vaccine – Early Results from an Indian Study [Internet]. [n.d.]. Available from: https://www.hpvworld.com/articles/single-dose-ef-cacy-using-the-bivalent-hpv-vaccine-early-results-from-an-indian-study/.
- HPVWorld. Single-Dose Efficacy Using the Bivalent HPV Vaccine – Post Hoc Results from Two Phase 3 Randomized Clinical Trials: The Costa Rica Vaccine Trial and PA-TRICIA Trial [Internet]. [n.d.]. Available from: https://www.hpvworld.com/articles/single-dose-ef-cacy-using-the-bivalent-hpv-vaccine-post-hoc-results-from-two-phase-3-randomized-clinical-trials-the-costa-rica-vaccine-trial-and-patricia-trial/.
- HPVWorld. The Biological Rationale for a One-Dose HPV Vaccine [Internet]. [n.d.]. Available from: https://www.hpvworld.com/articles/the-biological-rationale-for-a-one-dose-hpv-vaccine/.
- NgobrolinHPV. MSD Indonesia Mengedukasi Masyara-kat Mengenai HPV dan Vaksinasi [Internet]. [n.d.]. Avail-able from: https://www.ngobrolinhpv.com/berita-msd-indonesia-mengedukasi-masyarakat-mengenai-hpv-dan-vaksinasi-sebagai-cara-pencegahannya-melalui-acara-ngobrolin-hpv-live.
- Quang C, et al. TitleUnknown. 2022. doi: 10.1016/j.it.2022.07.011
- Sehatnegeriku (Kementerian Kesehatan). 90 Persen Anak di Indonesia Ditargetkan Terlindungi dari HPV [Internet]. 2023 Nov 15. Available from: https://sehatnegeriku.kemkes.go.id/baca/rilis-media/20231115/1544263/90-persen-anak-di-indonesia-ditargetkan-terlindungi-dari-hpv/.
- Setiawan D, et al. TitleUnknown. 2024. doi: 10.1371/journal.pone.0290808
- Stanley M, et al. TitleUnknown. 2024. doi: 10.1016/j.vaccine.2024.01.046
- World Health Organization (WHO). WHO, UNFPA com-mend Indonesia’s efforts to eliminate cervical cancer; urge streamlined vaccine strategy and enhanced screening [Internet]. 2024 Nov 15. Available from: https://www.who.int/indonesia/news/detail/15-11-2024-who–unfpa-commend-indonesia-s-efforts-to-eliminate-cervical-cancer–urge-streamlined-vaccine-strategy-and-enhanced-screening.

Most Commented