COULD A TAILOR-MADE HIV VACCINE BE INDONESIA’S BREAKTHROUGH?
By: Amalia Rani Setyawati, Cintya Naya Danastri, Ivana Yulian
What’s this about?
HIV/AIDS continues to affect millions of people worldwide, and although antiretroviral therapy (ART) has transformed the disease into a manageable chronic condition, access to treatment remains uneven, especially in low- and middle-income countries like Indonesia. CRF01_AE is a frequent HIV subtype in Indonesia. Patients infected with CRF01_AE generally have faster disease progression and lower survival rates. Recent studies have also shown that CRF01_AE is associated with distinct clinical and immunological features, necessitating tailored approaches. Given this context, a low-cost, locally tailored vaccine would offer a powerful alternative.
In commemoration of HIV Vaccine Awareness Day on May 18, this edition highlights recent progress in HIV vaccine research, including an innovative approach developed specifically for Indonesia. A study by Khairunisa et al. introduced a multi-epitope vaccine (MEV) designed specifically for the CRF01_AE subtype using bioinformatics tools. Epitope-based vaccines rely on carefully selected viral fragments (antigens) that can effectively stimulate the immune system. This study aimed to reduce HIV-related morbidity and mortality in Indonesia by creating a vaccine that fits the genetic profile of the local population.
How was the study conducted?
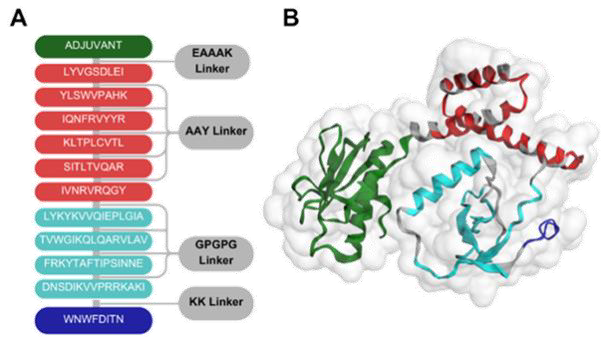
Khairunisa et al. began by analyzing over 900 genomic sequences of the HIV-1 CRF01_AE subtype to evaluate the mutation rates of various viral proteins. They found that the Pol protein had the lowest mutation rate, making it a stable and promising vaccine target. Although the Env protein showed a higher mutation rate, it was also selected due to its essential role in viral entry and immune recognition. From these two proteins, the researchers identified nine optimal epitopes—five for cytotoxic T cells (CTLs), four for helper T cells (HTLs), and one B cell epitope capable of inducing broadly neutralizing antibodies (bnAbs). Each epitope was evaluated for physicochemical properties such as solubility and structural stability. The selected epitopes were assembled into a multi-epitope vaccine construct with a modeled molecular structure and 3D conformation. Researchers then simulated the vaccine’s interaction with immune components using computational tools.
What did the study find?
The study identified the critical epitopes for a subtype of HIV-1, which is prevalent in Indonesia and Southeast Asia. The structure of the designed vaccine is shown in Figure 1. The selected epitopes demonstrated high antigenicity and immunogenicity scores, indicating their potential to elicit robust humoral and cellular immune responses, essential for long-lasting immune protection. The final MEV construct consisted of 272 amino acids and was predicted to be non-allergenic, with high thermal stability and strong interaction capabilities.
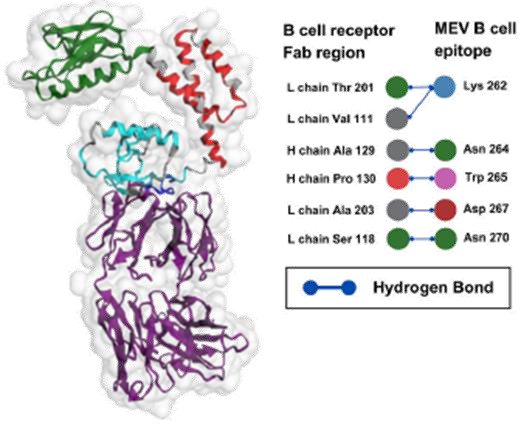
The fragment antigen-binding (FAB) region is the part of an antibody that specifically binds to antigens. Molecular docking results between the MEV construct and the FAB region demonstrated strong and specific interactions, involving 10 hydrogen bonds at the binding interface (Figure 2). These hydrogen bonds, essential for stabilizing protein-protein interactions, underscored the MEV’s potential to trigger robust and targeted immune responses. While the FAB region exhibited notable structural stability, the MEV construct showed greater flexibility, likely facilitating adaptive binding. This combination of MEV flexibility and stable complex formation with the FAB region suggests a promising design for effective vaccine development.
Immunogenicity assessments of the MEV construct revealed a robust antigen-specific immune response over a 35-day simulation period. Antigen levels peaked within the first five days before declining, while antibody titers progressively increased, reaching a maximum around day 15.2. These patterns indicated early immune activation followed by a sustained humoral response, supporting the construct’s potential efficacy. Cytokine profiling also showed a pronounced immunological response, further reinforcing the MEV’s capacity to stimulate both arms of the immune system.
Why does this matter?
Researchers have struggled to develop HIV-1 vaccines that elicit antibodies capable of neutralizing the virus’s diverse strains because of its extensive genetic diversity, high mutation rates, and rapid replication. This study presents a novel, Indonesia-specific vaccine strategy that addresses these obstacles by targeting the predominant CRF01_AE subtype.
Structural analysis of the MEV construct revealed high stability, solubility, and optimal exposure of antigenic components, all critical for effective immune recognition. This structural integrity is expected to be maintained during storage, transport, and administration, ensuring the vaccine’s effectiveness in real-world conditions. Complementary molecular docking and molecular dynamics simulations confirmed strong, stable interactions between the MEV and the FAB. Furthermore, immunogenicity assessments demonstrated significant and sustained antibody responses and diverse cytokine profiles, reflecting both immediate and long-term immune activation.
Any limitations?
While the study presents promising findings, it was conducted entirely using computational (in silico) methods, meaning that none of the predictions have been validated through laboratory experiments, animal studies, or human clinical trials. As such, the success of the MEV depends on how well it performs in real biological conditions. However, translating in silico results into in vivo outcomes is complex. Preclinical testing often relies on animal models, particularly non-human primates and simian-human immunodeficiency virus models, due to their genetic and immunological similarities to humans. Yet even minor differences between these models and human biology have caused many vaccine candidates that showed promise in animals to fail in human trials.
Moreover, despite targeting conserved viral regions, HIV’s high mutation rate and ability to evade immune responses remain significant hurdles. The risk of immune escape variants persists, even within a single subtype like CRF01_AE. Other biological challenges include the dynamic and diverse nature of HIV RNA, the virus’s replication within host immune cells, the need for highly specific neutralizing antibody responses, and a limited understanding of immune system-virus interactions. These complexities highlight the need for ongoing genetic surveillance and comprehensive experimental validation before the vaccine can move forward.
What’s next?
The next step is to test the vaccine design in real-world settings. Researchers need to start with lab experiments (in vitro) to check how well the epitopes bind to human immune proteins. Animal studies (in vivo) should be done to see if the vaccine can trigger an immune response and if it is safe. If those steps go well, the vaccine can move on to clinical trials in humans. It’s also important to monitor if the virus changes over time and update the vaccine design if needed. Researchers might add more epitopes or focus on highly conserved regions targeted by known bnAbs to make it even more effective.
Indonesia has never conducted an HIV vaccine trial in humans, while Thailand has been a leading site for HIV vaccine research in Southeast Asia. The VAX003 trial (2006) tested a gp120-based vaccine in people who inject drugs but failed to prevent HIV infection and had no significant impact on viral load, CD4+ T-cell counts, or disease progression. The RV144 trial (2003–2009), involving over 16,000 participants, was the first to demonstrate partial protection, with 60% efficacy at 12 months, declining to 31.2% by 3.5 years. More recently, the AP-PROACH trial (2015–2019), conducted in multiple countries, including a small cohort in Thailand (~58 participants), evaluated a mosaic-based HIV vaccine regimen. The vaccine was safe and well-tolerated, and all participants developed HIV-specific antibody responses and T-cell activation. Although the trial did not assess protection against infection, its findings supported the advancement to larger efficacy trials.
Finally, this study from Indonesia is a good starting point. If followed up with proper testing, it could lead to a vaccine designed for the specific HIV subtype in Indonesia. With continued research and collaboration, such efforts hold the potential to bring new hope in preventing HIV not only nationally, but also across the Southeast Asian region. Held annually on May 18, HIV Vaccine Awareness Day commemorates U.S. President Bill Clinton’s 1997 declaration that “only a truly effective, preventive HIV vaccine can limit and eventually eliminate the threat of AIDS.”
Article source:
Khairunisa SQ, Rachman BE, Nasronudin, Fahmi M, Di-nana IA, Ito M. Designing a multi-epitope vaccine targeting the HIV-1 subtype CRF01_AE in Indonesia. Computers in Biology and Medicine. 2025 Mar;187:109758.
References:
- Bbosa N, Kaleebu P, Ssemwanga D. HIV subtype diversity worldwide. Current Opinion in HIV and AIDS. 2019 May;14(3):153–60.
- Ueda S, Witaningrum AM, Khairunisa SQ, Kotaki T, Nasronudin, Kameoka M. Genetic Diversity and Drug Resistance of HIV-1 Circulating in North Sulawesi, Indonesia. AIDS Research and Human Retroviruses. 2019 Apr;35(4):407–13.
- Kim J, Vasan S, Kim JH, Ake JA. Current approaches to HIV vaccine development: a narrative review. J Int AIDS Soc. 2021 Nov;24(S7):e25793.

Most Commented