MONKEYPOX 101
By: Yan Mardian
First described in 1958, the human monkeypox virus (hMPXV) is a neglected zoonotic pathogen closely associated with the smallpox virus. Medical and public health officials are concerned—and puzzled—by the increasing number of confirmed monkeypox cases in countries outside central and western Africa, where the virus is endemic. Monkeypox is endemic in 10 countries in West and Central Africa, with dozens of cases this year in Cameroon, Nigeria, and the Central African Republic (CAR). The Democratic Republic of the Congo (DRC) has by far the highest-burden, with 1284 cases in 2022 alone. Those numbers are almost certainly underestimates. In the DRC, infections most often happen in remote rural areas; in the CAR, armed conflict in several regions has limited surveillance.
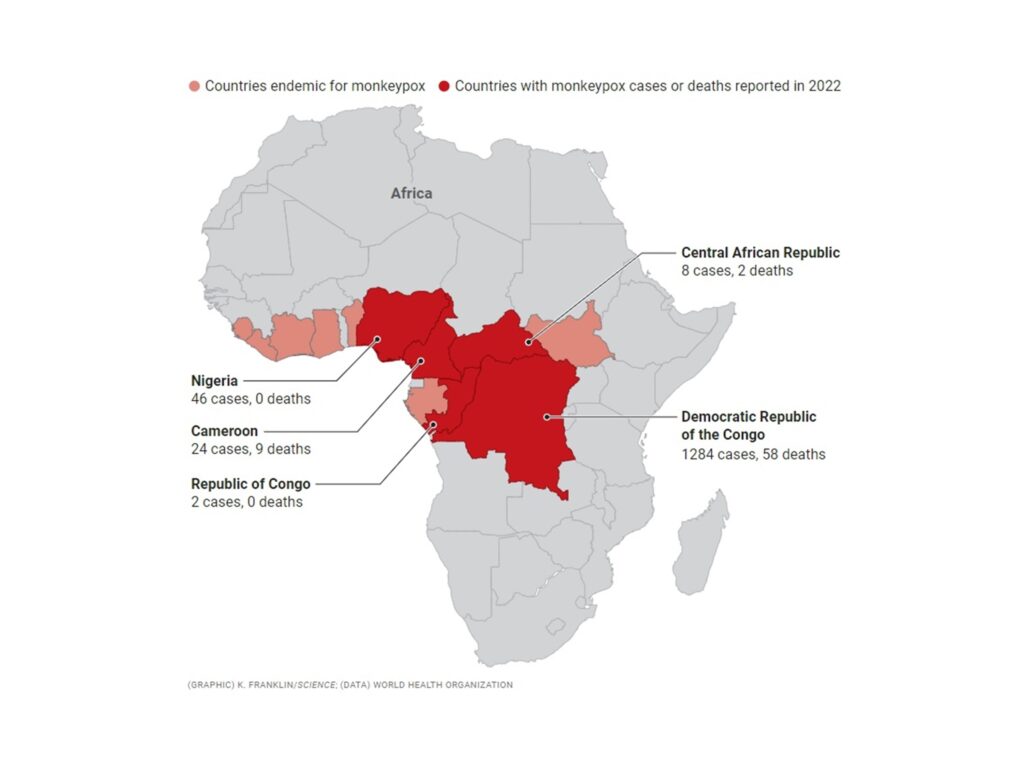
Figure 1. Endemic country for monkeypox. The virus infects squirrel, rat, and shrew species in at least 10 countries in West and Central Africa and occasionally jumps into the human population. So far this year, five countries have reported human cases.
As monkeypox stokes here-we-go again fears in a pandemic-weary world, some researchers in Africa are having their own sense of déjà vu. Another neglected tropical disease of the poor gets attention only after it starts to infect people in wealthy countries. On the 7th of May 2022, the UKHSA (United Kingdom Health Security Agency) confirmed the first case of the human Monkeypox virus (hMPXV) in a case travelling back from Nigeria. The patient had developed rashes few days ago before travelling to UK but presented to the hospital on the day of his arrival in UK. A reverse transcriptase polymerase chain reaction (RT-PCR) on a vesicular swab was performed and hMPXV infection was confirmed. Now, the fire is spreading. Since 1 January 2022, cases of monkeypox have been reported to WHO from 42 Member States across five WHO regions (the Regions of the Americas, Africa, Europe, Eastern Mediterranean, and Western Pacific).
In the past 5 years, scientists have confirmed only 8 cases where travelers carried monkeypox to countries outside Africa, including 2 cases last year in the US. Each was linked to a person who had recently spent time in Nigeria, a country that experienced a resurgence inmonkeypox starting in2017. In those cases, the human-to-human spread was limited; 2 family members became infected in one instance, according to the World Health Organization (WHO). One health care worker who had contact with contaminated bedsheets was infected in another case, report experts in an article published in the CDC’s Emerging Infectious Diseases. As of 15 June, a total of 2103 laboratory confirmed cases and one probable case, including one death, have been reported to WHO. The outbreak of monkeypox continues to primarily affect men who have sex with men who have reported recent sex with new or multiple partners. And unlike the previous cases discovered outside Africa, the current outbreaks have occurred in people with no travel history, suggesting that human-to-human transmission is driving the spread. Despite the increase in cases and human-to-human transmission, the risk to the general public remains low, according to a briefing by the WHO.

Figure 3. Geographic distribution of cases of monkeypox reported to WHO, between 1 January and 15 June 2022, (n=2103).
- Virology of MPX
Human monkeypox virus (hMPXV) is a ds DNA virus (~197 kb) of the Orthopoxvirus genus of the Poxviridae family. The subset includes Smallpox (variola), Vaccinia, and Cowpox viruses. hMPXV is a 200 to 250 nm large, brick shaped, enveloped, cytoplasmic virus that binds to glycosaminoglycans to enter the host cells. As an enveloped virus, it has been postulated to alternatively employ the classical apoptotic mimicry mechanism for entry in the host cells.

Figure 4. Schematic (left) and electron microscope image (right) depict monkeypox virus particles. On the left are mature, oval-shaped virus particles; on the right are crescent and spherical immature viruses
The virus got its name after it was first isolated in 1958 from smallpox-like vesiculopustular lesions amongst the captive imported monkeys (Java macaques) at the State Serum Institute in Copenhagen, Denmark. The monkeys were reported to suffer from a spontaneous outbreak of fever and rash. Over the next few years, similar outbreaks were reported in monkeys elsewhere. In 1966, the virus was identified as the causative agent behind a widespread outbreak at a zoo in Rotterdam. The virus was believed to have first affected the South American giant anteaters before spreading to various species of apes and monkeys. However, it has only been isolated from a wild monkey—in Africa—once. It appears to be more common in squirrel, rat, and shrew species, occasionally spilling over into the human population, where it spreads mainly through close contact, but not through breathing. Isolating infected people typically helps outbreaks end quickly.
Human disease was first identified in 1970 in a 9-month-old boy in the Democratic Republic of the Congo and since then most cases have been reported across Central and West Africa. The hMPXV has two described strains – the Central African/Congo Basin (CB) and West African (WA) strains. Historically, the CB clade appears to be more virulent, with a case fatality ratio (CFR) ranging from 1% to 10%, whilst the WA clade is associated with an overall lower mortality rate of < 3%. Recent data for the latter report a CFR of 1.4%. It is important to note that mortality in different settings may differ substantially. Genomic comparative studies have revealed a 0.55-0.56% nucleotide difference between the two strains, with the CB strain possessing 173 functional unique genes compared to 171 of the WA strain. Amongst the virulence genes, 53 out of 56 were found in both strains and showcased 61 conservative, 93 non-conservative, and 121 silent amino acid changes.
The differences in virulence between the two strains has been postulated to stem from the differences in the gene orthologs BR-203 (virulence protein), BR-209 (IL-1β binding protein), and COP-C3L (inhibitor of complement enzymes). Other candidate gene orthologs include the WA strain. specific COP-A49R (unknown function) and COP-A52R (Bifunctional Toll-IL-1-receptor protein). In terms of CB strain specific orthologs, candidate include BR-19 and BR-20 (unknown function). Another crucial gene responsible for difference in virulence in strains is the D14R gene coded inhibitor of complement-binding protein (MOPICE), an important anti-inflammatory factor which is absent from the hMPXV WA strain. However, these genes are not the only factors responsible for virulence, with many more candidates yet to be identified.
Recently, four genomes of the hMPXV isolated during the 2022 outbreak (Germany, USA, Portugal, and Belgium) were published online by various groups of researchers. The 2022 outbreak sequences are a part of a distinct cluster from 2022 within the West African clade. Limited sampling and sequencing of MPXV over the years makes it difficult to generate a hypothesis on the source of introduction for this outbreak. Analysis of the genomes preliminarily hint at very strong bias in mutations of bases Guanine (G) to Adenine (A) and Cytosine (C) to Thymine (T). The enzyme APOBEC3 (Apolipoprotein B Editing Complex), a cytidine deaminase, has been postulated to be responsible for these mutations. A genomic comparison from viral isolates from 2015 to 2022 showed a 30-T base long sequence in the middle of the viral genome, the role of which is yet to be determined.
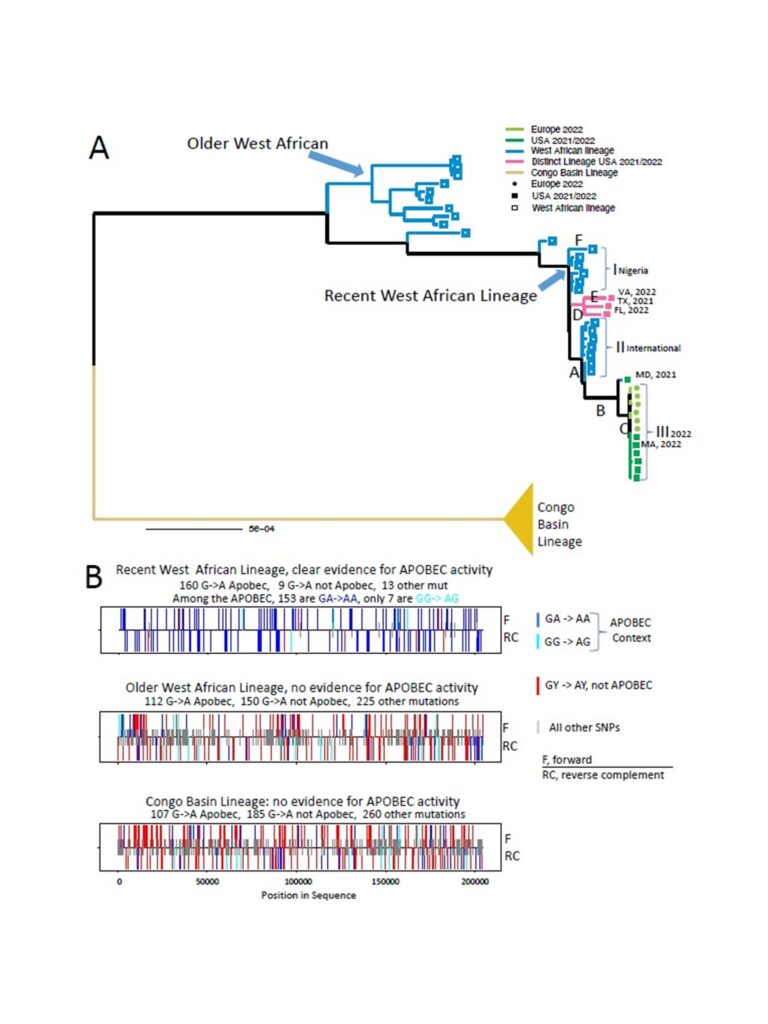
Figure 5. Analysis of APOBEC3 motif mutations in the West African MPXV lineage.
2. Natural History
The incubation period of MPX is usually 6 to 13 days following exposure but can range from 5 to 21 days (10). Although most people recover within weeks, severe complications and sequelae have been reported to be more common among those unvaccinated for smallpox compared with those vaccinated (74% vs 39.5%). It is unclear if there is waning immunity to smallpox vaccination over time; however, studies indicate that smallpox vaccination is approximately 85% effective in preventing MPX. Since prior smallpox vaccination may result in a milder disease course, it is important to ascertain vaccination status in any person exposed to MPX. Evidence of prior vaccination against smallpox can typically be found as a scar on the upper arm. Individuals over 40 to 50 years of age (depending on the country) may have been vaccinated against smallpox prior to cessation of global smallpox vaccination campaigns after the WHO declared eradication of the disease in 1980. Additionally, some laboratory personnel or health workers may have received the vaccine.
To date, most reported deaths have occurred in young children and immunocompromised individuals, such as those with poorly controlled HIV. A recent study from the Democratic Republic of the Congo reported that in a cohort of 216 patients, there were three deaths in patients < 12 years of age. When compared with survivors, patients with fatal disease had higher MPX viral DNA in blood, maximum skin lesion count, and day of admission AST and ALT values.
3. Signs and Symtoms
MPX can cause a range of clinical signs and symptoms. The initial phase of clinical illness typically lasts 1 to 5 days, during which time patients may experience fever, headache, back pain, muscle aches, lack of energy and lymphadenopathy – which is a distinctive feature of this disease. This is followed by a second phase, which typically occurs 1 to 3 days after fever subsides with the appearance of a rash. The rash presents in sequential stages – macules, papules, vesicles, pustules, umbilication before crusting over and desquamating over a period of 2 to 3 weeks. The lesions range in size from 0.5 to 1 cm in diameter and from a few to several thousand in number. The eruption tends to be centrifugal, starting on the face and extending towards the palms and soles of the hands and feet, and can involve the oral mucous membranes, conjunctiva, cornea and/or genitalia. Observations from current outbreaks in European and North American countries describe lesions starting in the genital area, but more information is needed.
Patients may develop lymphadenopathy – which was described in 98.6% of a cohort of over 200 patients with MPX in the Democratic Republic of the Congo. Oral ulcers are common and may affect a patient’s ability to eat and drink leading to dehydration and malnutrition. Inflammation of the pharyngeal, conjunctival and genital mucosae may also occur. A recent large prospective observational study describing the natural history of 216 patients with MPX in the Democratic Republic of the Congo described the most common clinical symptoms to be rash (96.8%), malaise (85.2%) and sore throat (78.2%). The most common findings on physical examination were the classic MPX rash (99.5%); lymphadenopathy (98.6% – the cervical region was most frequently affected [85.6%], followed by the inguinal region [77.3%]); and mouth/throat lesions (28.7%).
Though uncommon, patients with MPX may develop severe and life-threatening complications. For example, the confluence of skin lesions are susceptible to bacterial skin and soft tissue infections such as cellulitis, abscesses, necrotizing soft tissue infections requiring meticulous local wound care; subcutaneous accumulation of fluid in the crusting phase leading to intravascular depletion and shock; and exfoliation resulting in areas of skin that may require surgical debridement and grafting. Other rarer complications include severe pneumonia and respiratory distress, corneal infection which may lead to vision loss, loss of appetite, vomiting and diarrhoea which may lead to severe dehydration, electrolyte abnormalities and shock, cervical lymphadenopathy which may lead to retropharyngeal abscess or respiratory compromise, sepsis, septic shock, and, encephalitis and death. Small studies looking at laboratory abnormalities in patients with MPX indicate that leucocytosis, elevated transaminases, low blood urea nitrogen and hypoalbuminaemia were common features during illness, and that lymphocytosis and thrombocytopenia were seen in more than one-third of patients evaluated.
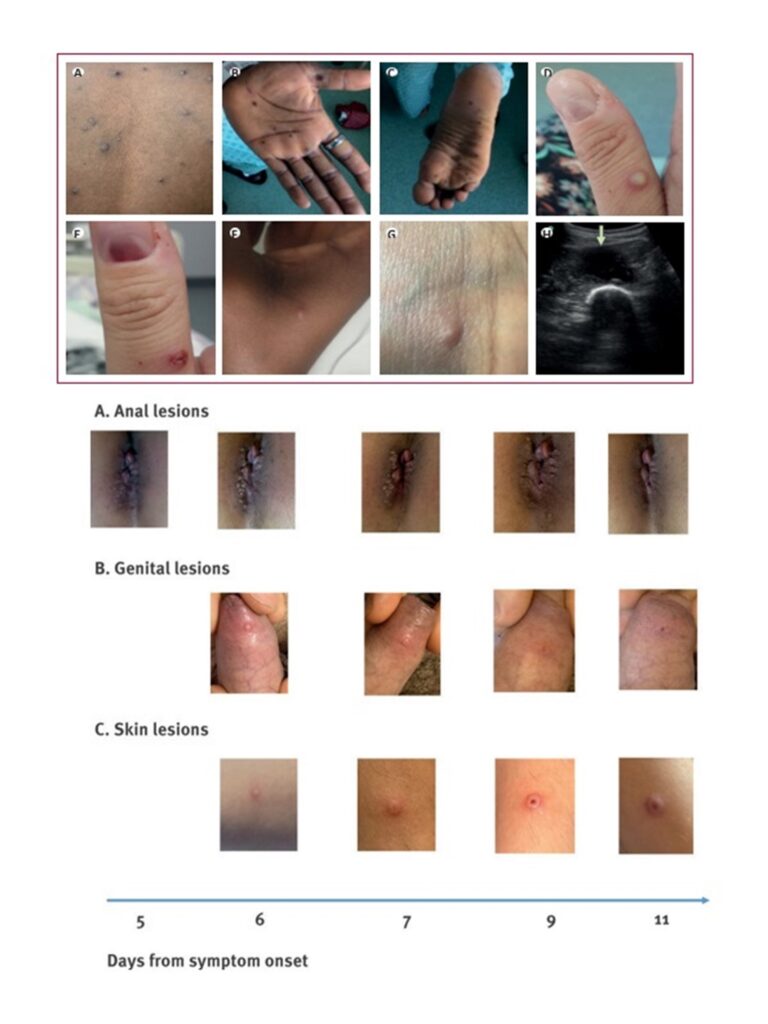
Figure 6. (above) Skin and soft tissue manifestations; and (below) genital manifestation of monkeypox
4. Transmission and viral shedding
Despite decades of circulation in animals with occasional spread to humans, there are limited data available describing transmission and viral shedding of MPX. Available information supports that transmission can occur from animal to human, human to human and from contaminated environments to humans. To date, most information is available from countries in West and Central Africa and less from areas in other WHO regions. MPX virus is transmitted from infected animals to humans via indirect or direct contact. Transmission may occur from bites or scratches, or during activities such as hunting, skinning, trapping, cooking, playing with carcasses, or eating animals, such as non-human primates, terrestrial rodents, antelopes and gazelles, and tree squirrels. The extent of viral circulation in animal populations is not entirely known and further studies are underway.
Human-to-human transmission can occur through direct contact with infectious skin or mucocutaneous lesions, this includes face-to-face, skin-to-skin, mouth-to-mouth or mouth-to-skin contact and respiratory droplets (and possibly short-range aerosols requiring prolonged close contact). The virus then enters the body through broken skin, mucosal surfaces (e.g. oral, pharyngeal, ocular and genital), or via the respiratory tract. The infectious period can vary, but generally patients are considered infectious until skin lesions have crusted, the scabs have fallen off and a fresh layer of skin has formed underneath. Transmission can also occur from the environment to humans from contaminated clothing or linens that have infectious skin particles (also described as fomite transmission). If shaken, these particles can disperse into the air and be inhaled, land on broken skin or mucosal membranes and lead to transmission and infection; one documented health worker infection has been published suggesting MPX virus transmitted through contact with contaminated bedding. Persistence of surrogate pox virus in the environment and on different types of surfaces has been found to last between 1–56 days depending upon the temperature and room humidity; however, there are currently limited data on surface contamination and fomite transmission, aside from contaminated linens. MPX are generally more resistant to environmental conditions and show high stability. No information on the presence of virus in wastewater.
A recent study published in May 2022 from the United Kingdom has reported on the clinical characterization, viral kinetics and polymerase chain reaction (PCR) positivity and response to antivirals in seven patients infected with MPX between 2018 and 2021. All seven patients had MPX viral DNA detected by PCR in skin lesions and in upper respiratory tract samples; six patients had DNA detected in blood; four patients had DNA detected in urine and one person had DNA detected in skin abscesses. Another recent study published in May 2022 on the clinical characterization of 216 patients diagnosed between 2007 and 2011 in the Democratic Republic of the Congo suggested that MPX viral DNA in blood and the upper respiratory tract may be detected prior to onset of rash and that peak viral load may occur very early in the disease course. Data also suggest the MPX scabs contain significant quantities of viral DNA until and including when they fall off and that it is higher than the levels found in the blood and throat. It should be noted that viral infectivity of specimens was not determined. At this time, the significance of these findings in relation to viral transmission and infectious period remains uncertain. More information is needed to better understand other possible modes of transmission and persistence via contact with other bodily fluids (such as breastmilk, semen, vaginal fluid, amniotic fluid or blood) and to better understand transmission by respiratory droplets and aerosols. In the current outbreak countries and amongst the reported MPX cases, transmission appears to be occurring primarily through close physical contact, including sexual contact (oral, vaginal and anal).
5. Differential diagnosis
The rash which develops in MPX may resemble other infectious diseases or other conditions, including varicella zoster virus (VZV, chickenpox), herpes simplex virus (HSV), primary or secondary syphilis, disseminated gonococcal infection (DGI), foot and mouth disease, chancroid, lymphogranuloma venereum (LGV), granuloma inguinale, molluscum contagiosum, measles, scabies, rickettsia pox, chikungunya, zika virus, dengue fever, vasculitis and other bacterial skin and soft tissue infections. Often, the rash caused by VZV can be confused with MPX but can be distinguished as the rash in varicella generally progresses quicker, is more centrally located than the centrifugal distribution of MPX, is in multiple stages of development (rather than the same stage as seen in MPX) and patients usually do not have lesions on their palms and soles. Additionally, patients with VZV typically do not have lymphadenopathy, which is a hallmark of MPX.
Despite the clinical differences between these two diseases, a study from the Democratic Republic of the Congo reported co-infection with MPX/VZV with an incidence of 10–13%. Patients with co-infection reported fatigue, chills, headache and myalgias. These individuals were less likely to report signs/symptoms of oral sores, axillary lymphadenopathy, cough or sore throat. Patients with co-infection had a higher lesion burden than seen with VZV alone but a lower rash burden than seen with MPX alone raising the suggestion that co-infection with these two viruses could modulate severity of the overall infection – an area for further investigation.

Figure 7. MPX surveillance, investigation and contract tracing
6. Diagnostic Test
The recommended specimen type for laboratory confirmation of monkeypox is skin lesion material, including swabs of lesion surface and/or exudate, roofs from more than one lesion, or lesion crusts. Swab the lesion vigorously, to ensure adequate viral DNA is collected. Both dry swabs and swabs placed in viral transport media (VTM) can be used. Two lesions of the same type should be collected in one single tube, preferably from different locations on the body and which differ in appearance.
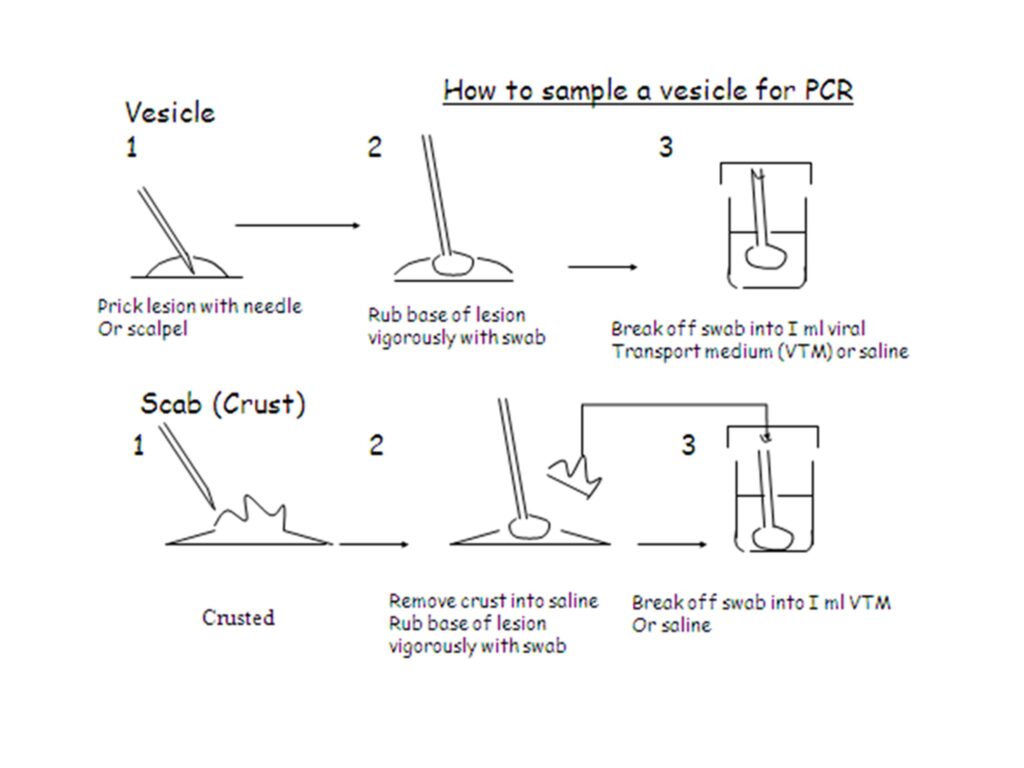
Figure 8. Guidance of specimen collection from lesion
Testing for the presence of MPXV should be performed in appropriately equipped laboratories by staff trained in the relevant technical and safety procedures. Confirmation of MPXV infection is based on nucleic acid amplification testing (NAAT), using real-time or conventional polymerase chain reaction (PCR), for detection of unique sequences of viral DNA. PCR can be used alone, or in combination with sequencing. Several groups have developed validated PCR protocols for the detection of OPXV and more specifically MPXV, some of which include distinction of Congo Basin and West African clades. Some protocols involve two steps, in which the first PCR reaction detects OPXV, but does not identify which species. This can then be followed by a second step, which can be PCR-based or utilize sequencing, to specifically detect MPXV. Before an assay is utilized to test human clinical specimens within a laboratory, it should be validated and/or verified within the laboratory by appropriately trained staff.

Figure 9. Testing algorithm for MPX virus
7. Treatment and vaccines
Many people infected with monkeypox virus have a mild, self-limiting disease course in the absence of specific therapy. However, the prognosis for monkeypox depends on multiple factors, such as previous vaccination status, initial health status, concurrent illnesses, and comorbidities among others. Currently there is no treatment approved specifically for monkeypox virus infections. However, antivirals developed for use in patients with smallpox may prove beneficial against monkeypox. The following medical countermeasures are currently available from the Strategic National Stockpile (SNS) as options for the treatment of monkeypox:
- Tecovirimat (also known as TPOXX, ST-246)
TPOXX is an antiviral medication that is approved by the United States Food and Drug Administration (FDA) for the treatment of smallpox in adults and children. Data are not available on the effectiveness of tecovirimat in treating monkeypox infections in people, but studies using a variety of animal species have shown that tecovirimat is effective in treating disease caused by orthopoxviruses. Clinical trials in people showed the drug was safe and had only minor side effects. US-CDC holds an expanded access protocol (sometimes called “compassionate use”) that allows for the use of stockpiled tecovirimat to treat monkeypox during an outbreak. Tecovirimat is available as a pill or an injection. For children who weigh less than 28.6 pounds, the capsule can be opened, and medicine mixed with semi-solid food.
- Vaccinia Immune Globulin Intravenous (VIGIV)
VIGIV is licensed by FDA for the treatment of complications due to vaccinia vaccination including eczema vaccinatum, progressive vaccinia, severe generalized vaccinia, vaccinia infections in individuals who have skin conditions, and aberrant infections induced by vaccinia virus (except in cases of isolated keratitis). CDC holds an expanded access protocol that allows the use of VIGIV for the treatment of orthopoxviruses (including monkeypox) in an outbreak. Data are not available on the effectiveness of VIG in treatment of monkeypox virus infection. Use of VIG has no proven benefit in the treatment of monkeypox and it is unknown whether a person with severe monkeypox infection will benefit from treatment with VIG. However, healthcare providers may consider its use in severe cases. VIG can be considered for prophylactic use in an exposed person with severe immunodeficiency in T-cell function for which smallpox vaccination following exposure to monkeypox virus is contraindicated.
- Cidofovir (also known as Vistide)
Cidofovir is an antiviral medication that is approved by the FDA for the treatment of cytomegalovirus (CMV) retinitis in patients with Acquired Immunodeficiency Syndrome (AIDS). Data is not available on the effectiveness of Cidofovir in treating human cases of monkeypox. However, it has shown to be effective against orthopoxviruses in in vitro and animal studies. CDC holds an expanded access protocol that allows for the use of stockpiled Cidofovir for the treatment of orthopoxviruses (including monkeypox) in an outbreak. It is unknown whether or not a person with severe monkeypox infection will benefit from treatment with Cidofovir, although its use may be considered in such instances. Brincidofovir may have an improved safety profile over Cidofovir. Serious renal toxicity or other adverse events have not been observed during treatment of cytomegalovirus infections with Brincidofovir as compared to treatment using Cidofovir.
- Brincidofovir (also known as CMX001 or Tembexa)
Brincidofovir is an antiviral medication that was approved by the FDA on June 4, 2021 for the treatment of human smallpox disease in adult and pediatric patients, including neonates. Data is not available on the effectiveness of Brincidofovir in treating cases of monkeypox in people. However, it has shown to be effective against orthopoxviruses in in vitro and animal studies. CDC is currently developing an EA-IND to help facilitate use of Brincidofovir as a treatment for monkeypox. However, Brincidofovir is not currently available from the SNS.
Table 1. Summary of Regulatory Licencing Antivirals for Monkeypox
| Tecovirimat | Brincidofovir | Cidofovir | |
| Treatment dose, route, duration (adults) (65,66,71,73,76) | Dose Oral 600mg PO every 12 hours Intravenous* 3 kg to < 35 kg: 6 mg/kg every 12 hours 35 kg to < 120 kg: 200 mg every 12 hours > 120 kg: 300 mg every 12 hours *Must be administered over 6 hours Duration 14 days | Dose Oral < 10 kg: 6 mg/kg 10–48 kg: 4 mg/kg > 48 kg: 200 mg (20 mL) Duration Once weekly for 2 doses, on days 1 and 8 | Dose Intravenous 5 mg/kg IV once weekly Must be given with oral probenecid: 2 grams 3 hours prior to each dose and 1 gram at 2 and 8 hours after completion of the infusion Must be given with at least 1 L of 0.9% normal saline over a 1–2 hour period before each infusion Duration Once weekly × 2 weeks, then once every other week (based on treatment for CMV retinitis) |
Treatment dose, route, duration (paediatrics) (65,66,71,73,76 | Dose Oral 13–25 kg: 200 mg every 12 hours 25–40 kg: 400 mg every 12 hours > 40 kg: 600 mg every 12 hours Intravenous* 3–35 kg: 6 mg/kg every 12 hours 35–120 kg: 200 mg every 12 hours > 120 kg: 300 mg every 12 hours *Must be given over 6 hours Duration 14 days | Dose Oral < 10 kg: 6 mg/kg 10–48 kg: 4 mg/kg > 48 kg: 200 mg (20 mL) Duration Once weekly for 2 doses, on days 1 and 8 | Dose Intravenous 5 mg/kg IV once weekly Must be given with oral probenecid: 2 grams 3 hours prior to each dose and 1 gram at 2 and 8 hours after completion of the infusion Must be given with at least 1 L of 0.9% normal saline over a 1–2 hour period prior to each infusion. Duration Once weekly × 2 weeks, then once every other week (based on treatment for CMV retinitis) |
| Dosage forms and strength | Capsules: 200 mg orange and black (65) Intravenous: IV injection single-dose 200 mg/20mL (71) | Tablets: 100 mg, blue, oval shaped (73) Suspension: lemon-lime flavoured suspension containing 10 mg/mL (73) | Intravenous: supplied as single-use vials 75 mg/mL for intravenous infusion (76) |
| Use in pregnancy | No data from the use in pregnant women (65,66) | Not recommended Administration to small animals resulted in embryotoxicity, decreased embryo-fetal survival, and/ or structural malformations. It is recommended to use an alternative therapy if feasible (73) | Pregnancy class C No adequate well controlled studies in pregnant women (76) |
| Use in breastfeeding | Unknown whether medicine or metabolites are excreted in human milk (65,66,70) | In studies with lactating rates, brincidofovir was detected in milk but not plasma of nursing pups (73) | Unknown (76) |
| PEP dose, route, duration (adult) | No data | No data | No data |
| Mechanism of action | Inhibits activity of the orthopoxvirus VP37 protein and inhibits viral envelope formation (65,69,70,72) | Inhibits polymerase mediated synthesis of DNA (73) | Inhibits DNA polymerase (79,80) |
| Licensed for smallpox | European Medicines Agency (2022)(65) US Food and Drug Administration (2021)(66) Health Canada (2021)(67) | US FDA (2021) (73) EMA (2016) | US CDC (EA-IND) |
| Licensed for monkeypox | European Medicines Agency (2022) (65,70) US CDC (EA-IND protocol) | US CDC (EA-IND protocol) | US CDC (EA-IND) |
Various smallpox vaccines, containing vaccinia virus, provide cross-protection against other orthopoxviruses (OPXV), including monkeypox, therefore national health authorities should conduct a risk assessment and consider whether arranging immunization for health care workers, including laboratory personnel, and other staff that are at risk of exposure to individuals or specimens with MPXV is required. Vaccination against smallpox was demonstrated through several observational studies to be about 85% effective in preventing monkeypox. Thus, prior smallpox vaccination may result in milder illness. Evidence of prior vaccination against smallpox can usually be found as a scar on the upper arm. Because the smallpox vaccine provides cross-protection from other OPXV, experts have suggested that the upward trend in monkeypox cases is due in part to the decline in smallpox vaccinations in the post eradication era.
A non-replicating vaccine consisting of the modified vaccinia Ankara strain known as MVA-BN was approved for prevention of smallpox (which was declared eradicated in 1980) in 2013. In 2019 it was also approved for the prevention of monkeypox by two stringent regulatory authorities. This vaccine can also be considered for prevention of monkeypox in the occupational setting. Some countries have maintained strategic supplies of older smallpox vaccines from the Smallpox Eradication Programme (SEP) which concluded in 1980. These first-generation vaccines held in national reserves are not recommended for monkeypox at this time, as they do not meet current safety and manufacturing standards. Many years of research have led to development of new and safer (second- and third-generation) vaccines for smallpox, some of which may be useful for monkeypox and one of which (MVA-BN) has been approved for prevention of monkeypox. The supply of newer vaccines is limited and access strategies are under discussion.
Table 2. Smallpox and monkeypox vaccine options
| Vaccine (Manufacturer) | Licensed for smallpox (country, type, date) | Licensed for monkeypox (country, type, date) | Considerations | Presentation | Injections materials |
| MVA-BN (Bavarian Nordic) 3rd generation | EU: Imvanex has been authorised under exceptional circumstances (2013) Canada: Full MA (2013) USA: Full MA (2019) | USA, full MA (2019) Canada, full MA (2019) | Very limited supply Liquid-frozen formulation, approved for use in the general adult population Two doses four weeks apart | Liquid frozen or lyophilized (freeze-dried) Single dose vials (Multidose vials possible) | Needle and syringe (sub-cutaneous administration) |
| LC16 (KM Biologics) 3rd generation | Japan – Full MA (1975) USA – EIND (2014) | No | Approved for use in infants and children (all ages) as well as adults (all ages) | Freeze-dried Multidose vials | Bifurcated needle |
| ACAM2000® (Emergent BioSolutions) 2nd generation | USA – Approved | USA – EIND for PEP | Approved for use in adults aged 18 – 64 years of age. Earlier production by Sanofi Pasteur approved in France. | Freeze-dried Multidose vials | Bifurcated needle |
| Vaccinia, various strains* from national production 1st generation | Various countries Various national production (SEP), held by various countries | No | Regular potency testing recommended | Liquid frozen or lyophilized vials or ampoules | Bifurcated needle |
Based on currently assessed risks and benefits and regardless of vaccine supply, mass vaccination is not required nor recommended for monkeypox at this time. Human-to-human spread of monkeypox can be controlled by public health measures including early case-finding, diagnosis and care, isolation and contact-tracing. All decisions around immunization with smallpox or monkeypox vaccines should be by shared clinical decision-making, based on a joint assessment of risks and benefits, between a health care provider and prospective vaccinee, on a case-by-case basis. Post-exposure prophylaxis (PEP): for contacts of cases, PEP is recommended with an appropriate second- or third-generation vaccine, ideally within four days of first exposure (and up to 14 days in the absence of symptoms), to prevent onset of disease. Pre-exposure prophylaxis (PrEP) is recommended for health workers at high risk of exposure, laboratory personnel working with orthopoxviruses, clinical laboratory personnel performing diagnostic testing for monkeypox, and outbreak response team members as may be designated by national public health authorities.
References:
- World Health Organization. Clinical management and infection prevention and control for monkeypox: interim rapid response guidance, 10 June 2022. World Health Organization; 2022.
- World Health Organization (17 June 2022). Disease Outbreak News; Multi-country monkeypox outbreak in non-endemic countries: Update. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393
- World Health Organization. Laboratory testing for the monkeypox virus: interim guidance, 23 May 2022. World Health Organization; 2022.
- WHO Technical brief (interim) and priority actions: enhancing readiness for monkeypox in WHO South-East Asia Region, 28 May 2022. https://cdn.who.int/media/docs/default-source/searo/whe/monkeypox/searo-mp-techbrief_priority-actions_300522.pdf?sfvrsn=ae7be762_1\
- Gigante CM, Korber B, Seabolt MH, Wilkins K, Davidson W, Rao AK, Zhao H, Hughes CM, Minhaj F, Waltenburg MA, Theiler J. Multiple lineages of Monkeypox virus detected in the United States, 2021-2022. bioRxiv. 2022 Jan 1.
- Noe S, Zange S, Seilmaier M, Antwerpen MH, Fenzl T, Schneider J, Spinner CD, Bugert JJ, Wendtner CM, Wölfel R. Clinical and virological features of first human Monkeypox cases in Germany.
- Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J. Clinical features and management of human monkeypox: a retrospective observational study in the UK. The Lancet Infectious Diseases. 2022 May 24.
- Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, D’Abramo A, Cicalini S, Lapa D, Pittalis S, Puro V. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Eurosurveillance. 2022 Jun 2;27(22):2200421.
- Cohen J. Global outbreak puts spotlight on neglected virus. Science (New York, NY). Jun. 2022 Jun 3;3(376):1032-3.
- Harris E. What to Know About Monkeypox. JAMA. 2022 May 27.
- Lansiaux E, Jain N, Laivacuma S, Reinis A. The Virology of Human Monkeypox Virus (hMPXV): A Brief Overview.
- WHO Vaccines and immunization for monkeypox: Interim guidance, 14 June 2022. https://apps.who.int/iris/bitstream/handle/10665/356120/WHO-MPX-Immunization-2022.1-eng.pdf
- Centers for Disease Control and Prevention. Interim clinical guidance for the treatment of monkeypox. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html

Most Commented