UPDATES FROM THE 7TH REPORT INTERNATIONAL ANNUAL MEETING IN GOA, INDIA
– FACING CHALLENGES TOGETHER TO CHALLENGE TUBERCULOSIS
By: Adhella Menur, Melinda Setiyaningrum
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), was the deadliest infectious disease until COVID-19 surpassed it in 2020. The COVID-19 pandemic disrupted the positive global trend of TB control, setting it back by roughly ten years. With the waning of the COVID-19 emergency and the rising incidence and death toll from TB, the latter is poised to reclaim its grim distinction. Multinational collaboration in research to achieve breakthroughs for TB elimination—spanning prevention, diagnosis, and treatment—is essential. Addressing this need, the National Institute of Allergy and Infectious Diseases (NIAID) established the Regional Prospective Observational Research in TB (RePORT) International network in 2013 to support the establishment of regional RePORT consortia in cooperation with host countries (https://reportinternational.org/). This platform facilitates future combined or comparative data analyses and serves as a vital resource for collaborations both within countries and internationally. Initially, Indonesia, coordinated by INA-RESPOND, along with India, South Africa, Brazil, and China joined the network. More recently, the Philipines, South Korea, and Uganda have come on board. The project has been implemented in two phases: Phase I (2013-2023, completed) and Phase II (2019-present).
The RePORT consortia are unified by a common data and specimen collection protocol. Each Re-PORT country focuses on bolstering local TB data and specimen repositories and related research. RePORT International has standard protocols for two study cohorts: Cohort A for active TB and Cohort B for household contact. Depending on their budgets, countries can participate in one or both cohorts. INA-RESPOND took part in Phase I, adopting Cohort A in the TRIPOD study (Tuberculosis Research of INA-RESPOND On Drug Resistance, NCT02758236). Annual meetings of RePORT Inter-national are convened to foster coordination among member countries, share updates in TB science, present junior investigators’ research, and engage in high-level discussions. This year, the 7th RePORT International Annual Meeting spanned four days in Cavelossim, Goa, India, starting with a pre-meeting on September 5 and the main sessions from September 6-8, 2023. INA-RESPOND delegated Adhella Menur, a TRIPOD investigator, and Melinda Setiyaningrum, the TRIPOD Data Manager, to represent RePORT Indonesia.
The pre-meeting centered on data harmonization across the member countries. With Phase I completed, the RePORT International Data Manager team has been engaged in streamlining and storing the extensive database. Efforts have been made to harmonize data from bacteriologically confirmed TB adult patients from Cohort A in support of the Re-PORT International project titled “Epidemiologic factors associated with TB treatment outcomes across RePORT International consortia.” This project aims to ascertain the impact of non-communicable diseases, such as prediabetes and diabetes, and communicable diseases like HIV on TB treatment outcomes and recurrence on both global and regional scales. For this initiative, the TRIPOD study contributed data on 312 bacteriologically confirmed TB cases. The team is currently revisiting and finalizing data harmonization, targeting a database lock in October 2023, analysis in December 2023, and manuscript drafting in Q1 of 2024.
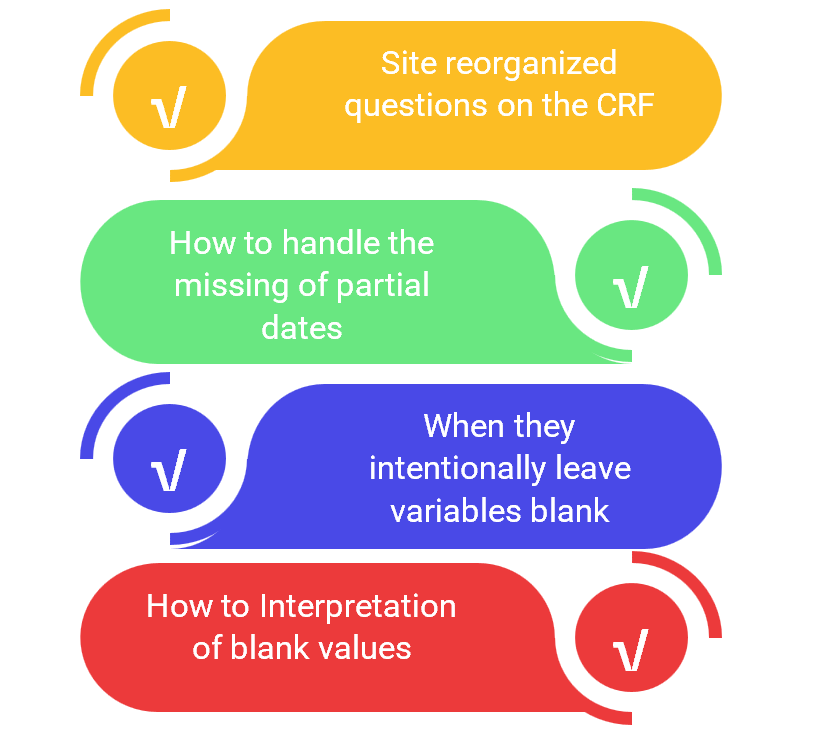
The RePORT International Data Manager team shared preliminary results and discussed challenges encountered during data harmonization. In Phase I, RePORT International merely provided guidelines for collecting study variables. Consequently, each country tailored its study Case Report Form (CRF), leading to varied data capture methods. Aligning CRF fields across countries proved challenging, with discrepancies most evident in areas like concomitant medications, non-TB disease history, and TB treatment compliance. Some CRFs also missed essential details or queries vital for effective data harmonization. For instance, the TRIPOD study’s CRF overlooked demographic details such as occupation, income, and living conditions. Challenges also arose in harmonizing co-infections, comorbidities, and non-TB medication data. For the latter, while some countries recorded information at different visits, others lacked such data altogether. Technical issues, including incomplete dates, varying formats, and ambiguous blank values, further complicated the process.
During the meeting, a productive discussion unfolded. The RePORT International team sought to address current challenges in data harmonization, draw lessons from them, and strategize for future projects, encompassing other cohorts and RePORT Phase II. For Phase II, collaboration is planned with the Frontier Science Foundation, a renowned non-profit research organization recognized globally for its trusted data management, statistical center, and publication support. Frontier Science will function as the central data-sharing hub, offering tools for secure data transfer. A specialized team will interpret the incoming data, ensuring thorough quality assurance and control, and addressing any issues directly with the data sender. The data will then be stored in a dedicated project database.
The second RePORT International project, titled “Analysis of host biomarkers associated with adverse TB treatment outcomes across RePORT Inter-national sites,” was also deliberated upon. The objective is to broaden the validation studies of host biomarkers linked to TB treatment failure and to pinpoint a “cure” biomarker through a discovery-driven method. The TRIPOD study provided data on 11 failure cases and 22 cure cases for analysis. The TRIPOD team has secured IRB approval for this initiative from the Persahabatan Hospital Ethics Committee and is in the process of preparing the MTA for PAXgene RNA and Plasma shipment. This project is especially advantageous as RePORT International facilitates laboratory and data analysis training in Central Labs located in the United States, Brazil, India, and South Africa in 2024, aiming to enhance laboratory capacity across the member countries.

The subsequent three days comprised the main meeting. It offered extensive insights into the operations of the RePORT International Coordinating Center (TB RiCC), updates on TB science, and potential collaborations with other networks, such as TB-SRN of IeDEA. The TB RiCC undergoes restructuring every five years. Representing the leadership group, Jerrold Ellner introduced the revamped organizational structure, TB RiCC 3.0, for 2023-2028. A notable addition to the organization is the Scientific Review Committee (SRC), entrusted with overseeing scientific activities within the RePORT network, like concept sheet reviews, grant proposal evaluations, and more. The TB RiCC 3.0’s roadmap encompasses updating the common protocol to reflect current knowledge, which includes integrating new procedures, visits, data, and specimens, conceptualizing RePORT Phase 3, introducing separate cohorts like diagnostics, early biomarkers, lung health, pediatrics, and studies on extrapulmonary TB, and drafting a Bylaws document that will detail regulations for RePORT projects. Ellner also showcased the current standing of the RePORT network, highlighting 32 study sites across six countries, encompassing 13,534 participants, 391,605 stored specimens, and over 140 associated publications, with a significant contribution from India, Brazil, and South Africa. He expressed optimism regarding the network’s continued growth and productivity.
The sessions updating TB science covered a spectrum from basic research, subclinical TB, post-TB lung health, to vaccines. These also included presentations by Junior Investigators from the Re-PORT countries network. Subclinical TB emerged as a focal point in the scientific discussions. This category denotes a condition caused by viable MTB that doesn’t manifest as clinical TB symptoms but triggers detectable abnormalities through existing radiological or microbiologic assays. The progression from subclinical TB to active TB varies greatly, ranging from approximately 5-7 months to possibly 16 months. Intriguingly, many cases resolve spontaneously, while some individuals remain asymptomatic for extended periods (yet might be infectious). Sub-clinical TB is believed to account for nearly half of all transmission, and treatments might curb this spread. A study from Yogyakarta, Indonesia (Ananda NR, Trop. Med. Infect. Dis. 2023, 8(9), 447; https://doi.org/10.3390/tropicalmed8090447) disclosed that of the 47,735 individuals who participated in the active case finding (ACF) program via chest X-rays, 393 were diagnosed with TB. Remarkably, 176 of these 393 cases (44.8%) were asymptomatic, with 52 of the 176 (29.5%) being bacteriologically confirmed. This underscores the pressing need to comprehend and formulate data-driven guidelines for diagnosing and managing subclinical TB.
A significant area of scientific interest was lung health following TB treatment. According to Dodd et al. in Lancet Infect Dis., as of 2020, an estimated 155 million TB survivors were alive worldwide. The pooled standardized mortality ratio for TB survivors stood at 2.9 compared to individuals without TB. TB survivors face risks of TB recurrence and other diseases, including post-TB lung disease, cardiovascular disease, lung cancer, and autoimmunity. Post-TB lung disease is a chronic respiratory condition, with or without symptoms, largely attributed to prior TB. This ailment can lead to COPD, restrictive ventilatory defects, aspergilloma, exacerbations of bronchiectasis, and pulmonary hypertension. Overcoming TB is a prolonged journey that doesn’t conclude with the final dose of anti-TB medication. Continuous monitoring after TB treatment is essential to enhance survivors’ health and quality of life. There’s an anticipation for studies focusing on lung health post-TB treatment, which would delve into the degree of lung injury, markers, prevention, and management.
Updates from the VPM1002 study by the Serum Institute of India PVT. LTD., concerning a next-generation BCG vaccine, were also noteworthy. VPM1002 is a genetically modified BCG vaccine, promising enhanced immune activation and greater safety compared to traditional BCG. The vaccine successfully cleared phase I clinical trials in Germany and South Africa, proving its safety and immunogenicity in young adults. It was also validated in a phase IIa randomized clinical trial involving healthy South African newborns and a phase IIb trial with both HIV-exposed and unexposed infants. Subsequent trials are underway, including a phase III efficacy study in newborns to prevent MTB infection, trials in pulmonary TB patients post-successful TB treatment to avert TB recurrence, and in healthy household contacts of recently diagnosed sputum
positive TB patients to guard against MTB infection.
Six Junior Investigators, three from RePORT India, two from RePORT Brazil, and one from RePORT South Africa, showcased their research using data and specimens from their respective countries. These nations have matured in TB research, boasting advanced laboratory testing and academic collaborations. The presentations covered a range of topics, from TB recurrence, the influence of pre-existing nutritional status on TB severity, to the pharmacokinetics of Moxifloxacin in MDR-TB patients. The inclusion of Junior Investigator presentations is a staple at the RePORT annual meetings, aiming to foster scientific zeal among emerging researchers and ensure generational continuity. Re-PORT Indonesia aspires to send a larger contingent of young, gifted researchers to subsequent meetings, hoping for an even greater contribution.
On the concluding day of the RePORT Goa assembly, the network addressed updates and challenges encountered by each country. Representatives from every country provided a 20-minute brief on the status of ongoing projects, related scientific endeavors, SWOT analysis, and the potential to pursue studies on subclinical TB and its long-term ramifications. Representing Dr. Erlina Burhan for RePORT Indonesia, Adhella Menur showcased the TRIPOD study and associated publications. She elaborated on RePORT Indonesia’s endeavors in dissecting the TRIPOD data, pursuing collaborations, securing funding, and fortifying laboratory capabilities. A noteworthy collaboration involved fungal experts Retno Wahyuningsih and David W. Denning, culminating in the publication titled “The seroprevalence of anti-Histoplasma capsulatum IgG among pulmonary TB patients” in the PLOS NTD Journal. Sadly, several attempts to secure TB research grants were unsuccessful. As part of their laboratory capacity-building efforts, the INA-RESPOND Reference Laboratory has been actively performing TB tests on specimens from the TRIPOD repository, utilizing techniques like whole genome sequencing with Ox-ford Nanopore’s MinION and Sanger sequencing. Adhella also presented RePORT Indonesia’s SWOT analysis, emphasizing the commitment of INA-RESPOND and its hospital networks to advance TB elimination. By contributing more to the RePORT International network and participating in TB studies, RePORT Indonesia is geared up to collaboratively combat tuberculosis.



TB RiCC 3.0 Pilot Proposal Program
The Request for Application (RFA) soon will be announced. This program will fund two TB proposals. It must include at least two RePORT networks using RePORT data and specimens—the application deadline in December 2023.
TB RiCC 3.0 Capacity Strengthening Working Group Programs
E-learning TB courses and a 2-year mentored Post-Doctoral Fellowship Program for early-stage investigators (MD and/or Ph.D with <10 years’ experience since recent graduation). The mentorship program will fund two fellows annually from RePORT countries network. Applications open in January 2024.

Most Commented